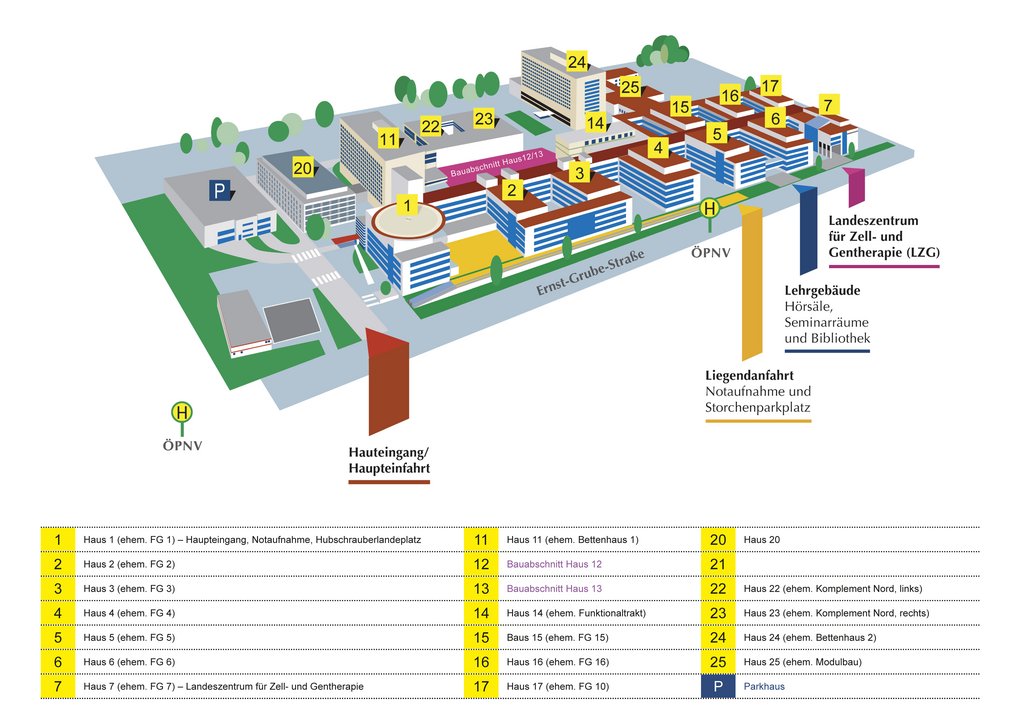
So finden Sie uns:
Universitätsklinikum Halle, Ernst-Grube-Straße 40, 06120 Halle
Anfahrt zum Hauptstandort Ernst-Grube-Straße 40
Anfahrt über A14 oder B100
Wenn Sie von der A 14 kommen, nutzen Sie bitte die Abfahrt Halle/Peißen auf der B 100 Richtung Innenstadt bis zum Dessauer Platz. Dort rechts halten auf die B 6 (Paracelsusstraße) Richtung Norden/Magdeburg. Nach 400 Metern halb rechts halten auf die Wolfensteinstraße. Die Straße ändert den Namen zu Große Brunnenstraße. Am Ende rechts abbiegen auf die Burgstraße, nach 140 Metern links auf die Kröllwitzer Straße. Sie fahren über die Brücke, fahren weiter auf der Dölauer Straße. An der Ampelkreuzung biegen Sie nach links auf den Brandbergweg. Von diesem biegen Sie an der zweiten Ampelkreuzung nach links in die Ernst-Grube-Straße.
Anfahrt über die B80
Über die B80 fahren Sie bis zum Rennbahnkreuz, dann über den Gimritzer Damm nach Norden. Sie biegen rechts in den Weinbergweg ein, dann links in die Ernst-Grube-Straße.
Parken
Am Standort Ernst-Grube-Str. steht ein kostenpflichtiges Parkhaus zur Verfügung.
Achtung Baustellen!
In der Stadt Halle wird viel gebaut. Bitte informieren Sie sich vor Ihrer Fahrt über mögliche Umleitungen zum Beispiel auf dem Baustellenkalender der Stadt Halle.
Der Ansprechpartner für das Parkhaus ist
A. Himmelreich GmbH & Co.KG
Im Zollhafen 24
50678 Köln
Frau Zschoche: Tel. 0176 504 649 48
Seit dem 24.04.2023 gilt ein neuer Fahrplan.
Für die Linien, die zu den Haltestellen "Heide - Universitätsklinikum" und "Kröllwitz" führen, gibt es folgende Änderungen:
Straßenbahnen:
Linie 4: wird zur Buslinie 4 und pendelt zwischen Rennbahnkreuz - Heide-Universitätsklinikum - Kröllwitz
Linie 5: fährt aus Bad Dürrenberg nur noch bis Berliner Brücke und weiter als Linie 12 nach Trotha (also nicht mehr nach Kröllwitz)
Linie 94: entfällt komplett
Linie: 2: fährt ab Rennbahnkreuz neu weiter bis Heide-Universitätsklinik und Kröllwitz
Busse:
Linie 4: wird zur Buslinie 4 und pendelt zwischen Rennbahnkreuz - Heide-Universitätsklinikum - Kröllwitz
Linie 34: fährt von Halle-Neustadt nach Heide-Universitätsklinikum
Linie 36: fährt von Zscherben nach Heide-Universitätsklinikum
Anreise
Fahrzeuge dürfen vor dem Haupteingang halten, um gehbehinderten Personen den Zugang zum UKH zu erleichtern.
Parkplätze
Ausgewiesene Behindertenparkplätze befinden sich im Parkhaus gegenüber dem Haupteingang.
Zugang
Das Klinikum ist barrierefrei zugänglich. Im Außenbereich gibt es Treppen und Rampen. Mit Fahrstühlen können alle Stockwerke erreicht werden.
Vom Haupteingang führt ein blinden- und sehbehindertengerechtes Wegeleitsystem rechterhand zur Zentralen Notaufnahme und linkerhand zur Cafeteria, zum zentralen Informationstresen und zur stationären Patientenaufnahme. Dort stehen Ihnen jeweils Mitarbeiterinnen und Mitarbeiter persönlich zur Verfügung.
Haupteingang
Der Zugang ist per Rampe oder Treppen möglich. Zudem existieren automatische Schiebetüren. Der Großteil der Ambulanzen und die Zentrale Notaufnahme befinden sich in der Ebene des Haupteingangs.
Eingang FG 5/6: Lehr- und Lerngebäude
Neben den Lehr- und Lerngebäuden erreichen Sie über diesen Eingang auch die Ambulanzen und Sprechstunden des Departments für Orthopädie, Unfall- und Wiederherstellungschirurgie. Der Zugang ist per Rampe und automatischer Schiebetür möglich.
Eingang LZG (Landeszentrum für Zell- und Gentherapie Halle)
Der Zugang erfolgt mittels Drehtür und automatische Schwenktür.
Rollstuhlgerechte Toiletten
Behinderten-WCs befinden sich in allen Gebäudeabschnitten am Hauptstandort:
- Bettenhaus I (innerhalb der Stationen)
- Bettenhaus II (auf dem Flur in der 1. und 2. Etage; innerhalb der Stationen der Dermatologie und Nephrologie)
- Komplement (innerhalb der Inneren Ambulanz und Physiotherapie, des Kreißsaals sowie der Viszeral- und Gefäßchirurgie und der Ambulanz für Neurologie)
- Funktionsgebäude 1 (innerhalb der Zentralen Notaufnahmen und Urologie)
- Funktionsgebäude 3 (innerhalb der Station Pädiatrie II)
- Funktionsgebäude 4 (innerhalb der Station Pädiatrie I und der interdisziplinären operativen Kinderstation
- Funktionsgebäude 10 (in unmittelbarer Nähe des Restaurants „Halle Blick“ und der Ambulanz für Orthopädie/ Unfall- und Wiederherstellungschirurgie)
- Funktionsgebäude 16 (innerhalb der Ambulanz für Nuklearmedizin)